Discover our Labs and Team in 190 seconds >
Quality, Responsiveness & Innovation
As a preclinical CRO, Atlantic Bone Screen is dedicated to optimize and accelerate the development of your compounds, identifying and characterizing the therapeutic potential of your drug candidates, nutraceuticals and biomaterials.
We focus on meeting the preclinical needs of our Partners and Customers, providing efficient support, relevant preclinical models and high value-added services, always keeping in consideration the regulatory requirements.
Let’s accelerate your development with our Team in a rigorous and collaborative relationship!
What our customers say about us?
20 years of expert services
Atlantic Bone Screen is a leading CRO (« Contract Research Organization ») specialized in high-quality preclinical evaluation services and non clinical studies.
Founded in 2005 from an academix lab expert in bone resorption and bone physiology, Atlantic Bone Screen offers a unique preclinical platform with a wide range of reliable in-vitro and in-vivo assays as well as histology and imaging services.

More than 2500 preclinical studies have been conducted by our Team since its creation. Research centers from the most important pharmaceutical and food industries (ingredients and nutraceuticals) as well as biomaterial companies and emerging biotech have put their trust in Atlantic Bone Screen.
Our company belongs to the regional network of biotechnology companies of the Atlanpole Biotherapies cluster. Atlantic Bone Screen has many partners and consultants providing complementary services, especially expert CRO’s member of AFSSI national association.
Atlantic Bone Screen is a registered as a service provider for research tax credit “CIR agreement” from the French Ministry of Research.
Available on different Marketplaces
 In order to adapt to our Customers’ purchase systems, we have opened our services through online marketplaces such as Scientist.com and Science Exchange.
In order to adapt to our Customers’ purchase systems, we have opened our services through online marketplaces such as Scientist.com and Science Exchange.
Our focus is your satisfaction
By parnering with us, you will discover our culture of expertise, committment and professionalism and our dedication to deliver reliable, robust and actionable results to help your projects meet regulatory milestones.
Numerous technical standards and very high levels of requirements are respected, taking into account scientific progress in many areas and the obligations and recommendations from all global health authorities.
In the last decades, we have been rewarded by positive testimonials from our Partners and Sponsors as shown by our satisfaction surveys that never fall below a 95% customer satisfaction rate.

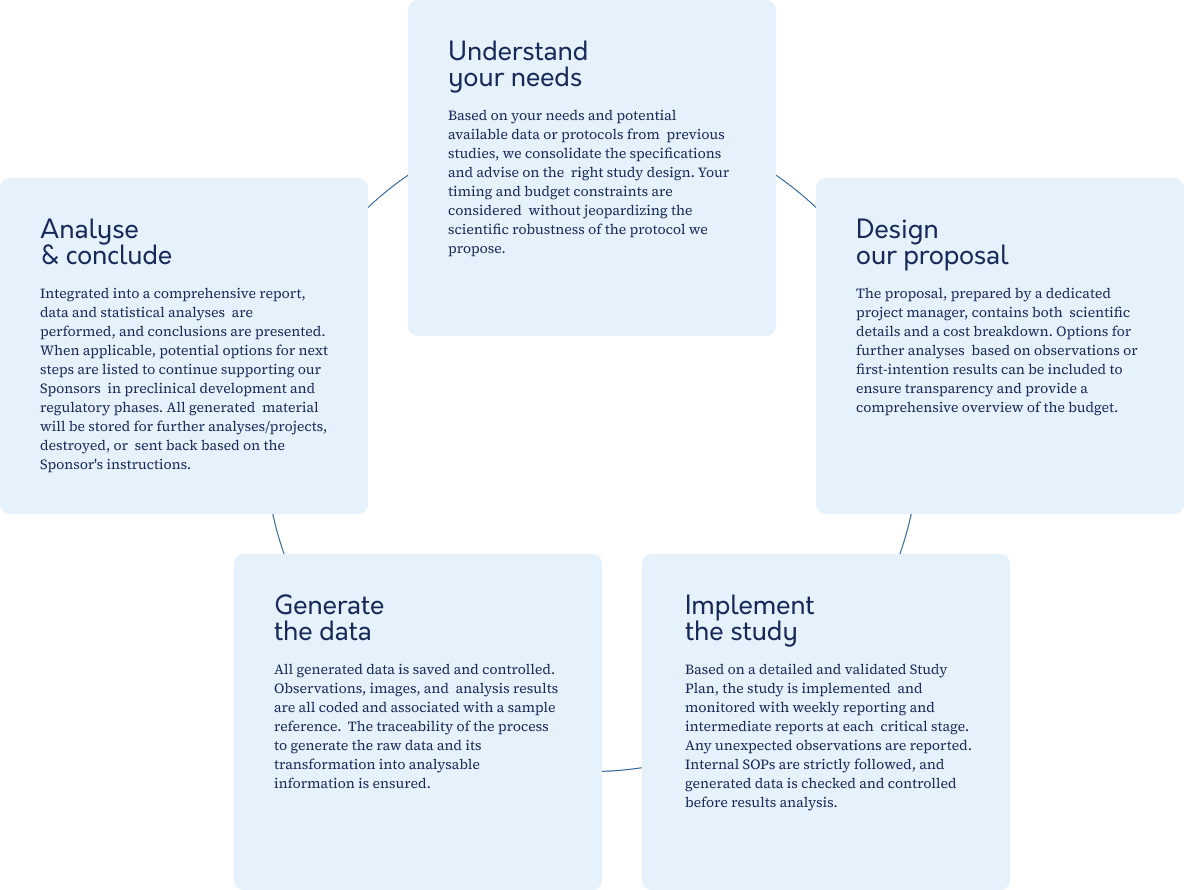
The stages of our collaboration
The way we work fully reflects our mission and values to always provide responsive, innovative and high quality support and services: from understanding your needs, we discuss and design customized protocols, conduct studies with close and weekly follow-up and, once the data are generated, we provide a complete report with statistical analyses, conclusions and options for further investigation.