Pathological models mimicking human and animal pathologies such as osteoporosis, osteoarthritis, rheumatoid arthritis...

Non-clinical testing of implantable biomaterials
Either for orthopaedics or dental applications or to improve skin health by treating wounds, boosting the appearance of the skin, medical device development and biomaterials research has shown a huge expansion in the last decades evolving from metal or ceramic or polymers to nanoparticles and “active” biomaterials.
“Materials scientists have been playing important roles in the design and manufacturing of orthopedic implants and tissue engineering scaffolds for several decades. However, until more recently, they have not had much involvement in cancer therapy” (source: The evolution of biomaterials research – Volume 16, Issue 11, Page 408–409 | Amir A. Zadpoor).
Our preclinical platforms offer comprehensive and scientifically validated solutions to evaluate the biocompatibility and efficacy of biomaterials. From in vitro cytotoxicity assays to advanced in vivo models, we provide tailored approaches for material characterization and development. From early-stage material screening to advanced histopathological evaluation, our models ensure reliable and actionable data to guide and accelerate the development of your mdecial devices.
Reliable in vitro Cytotoxicity Testing
We perform direct and indirect cytotoxicity assays in compliance with ISO 10993 standards to study biocompatibility, offering early-stage screening of biomaterials in non-GLP settings. Our capabilities include:
- Bone and joint cell assays using osteoblasts, chondrocytes, fibroblasts, and other relevant cell types for application in bone repair.
- Skin cell testing using keratinocytes and fibroblasts (use of L929 cells) for applications in dermatology and wound healing.
These assays ensure robust assessment of cellular responses, including viability, proliferation, and morphology, to help identify promising candidates.
Know more on our biocompatibility tests
In vivo implantation models
Atlantic Bone Screen also provides robust in vivo models (different implantation locations and times, various species from rodents to large animals, pathological and non-pathological…) in order to help Biomaterials researchers and industries in the evaluation of:
- Biocompatibility and immune response.
- Efficacy in promoting bone healing and tissue regeneration.
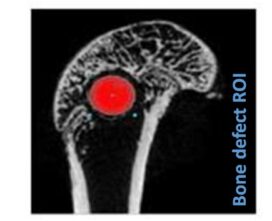
Advanced imaging tools, such as microCT and 3D reconstruction, provide high-resolution visualization of the implant site, enabling longitudinal studies of bone remodeling and material integration.
We thus provide results on biomaterials ability to become a bone substitute focusing on their osteoinductive and osteoconductive properties, or on their ability to become a skin substitute by promoting healing and regeneration for skin applications. We also provide results on the safety of the biomaterials developed.
The definition of the study design and the analyses are performed according to ISO 10993-5 (especially part 5 and part 6).
Know more on our in vivo implantation models
Comprehensive Histological Analysis
We offer a wide range of histology and histomorphometric services to evaluate tissue response at the implant site:
- Paraffin and resin embedding for precise sectioning and staining.
- Specialized techniques such as TRAP staining (to assess osteoclast activity) and Von Kossa-Von Gieson staining (to study mineralized tissue).
- Quantitative histomorphometry for detailed analysis of bone and tissue reconstruction.
- Histopathological evaluation by a veterinary pathologist for microscopic examination and evaluation of biocompatibility and integration of implants with surrounding tissue.
Know more on our histology for biomaterials >
Examples of studies performed:
- Biomaterials implantations into bone defect (femoral condyle, trabecular, cranial defects)
- Intra-muscular and subcutaneous implantation,
- Evaluation of osteoinductive properties, osteoformation, osteointegration and bone mineralization,
- Evaluation of thrombosis, local tissue effect and degradation, …
- Histopathology of biomaterials in situ.
Examples of tested biomaterials
Various type of biomaterials / medical devices have already been testing by our Team: cement, hyaluronic acid-based gel (dermal filler), powder, granules, membrane, hydrogel, bandage… Based on their form and size, study design and surgery can be adapted to ensure optimized protocol and avoid “leakage” once implanted.
In vitro and ex vivo models applied to dermocosmetics products to better understand mechanism of actions of compounds and products...
Preclinical models applied to inflammatory diseases to better understand mechanism of actions of compounds and treatment efficacy
Robust models on tumoral cell lines and in vivo oncological models to validate future drugs for bone cancer, bone metastases...