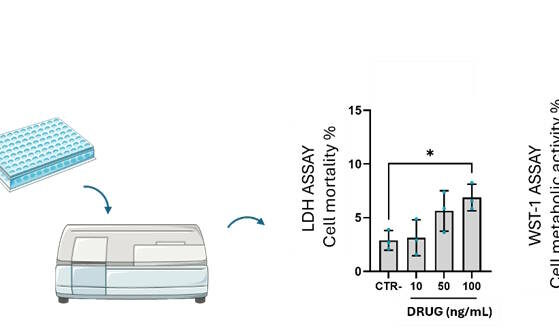
Biocompatibility refers to the harmonious interaction between a material and the biological environment in which it’s implanted. It encompasses:
- the material’s ability to coexist with living tissues without causing harm,
- its capacity to perform its intended function effectively within the body,
- the potential to support or enhance biological processes when required.
Assessing the biocompatibility of a medical device or of a new molecule requires in vitro and in vivo testing to obtain a complete understanding of its biological effects.
By choosing our in vitro testing services, you can accelerate your product development timeline, reduce costs associated with animal testing, and gain valuable insights into compounds translation.
Atlantic Bone Screen offers in vitro and in vivo biocompatibility testing services to support your medical device and pharmaceutical development needs.
By choosing our in vitro testing services, you can accelerate your product development timeline, reduce costs associated with animal testing, and gain valuable insights into compounds translation.
Our scientific team works closely with clients to design custom testing protocols tailored to your specific requirements and inspired by ISO-10993 standards.
Direct and indirect biocompatibility tests can be implemented depending on the nature of the tested biomaterial.
Our tests guarantee a robust, non-GLP evaluation, ensuring that you receive the most relevant and actionable data to support your product development.