Implant sites and biomaterials samples processing, staining and analyses - paraffin, frozen or resin process to cover biocompatibility and efficacy

Assess the effect of your biomaterials once implanted
Orthopedics surgeons are often confronted to bone substance loss in the case of bone fractures for example. In order to bridge it, they have at their disposal a large range of bone substitutes, from animal, human, mineral or synthetic origin. A bone substitute is a biomaterial or a tissue engineered product aiming at bridging and replacing bone loss and thus induce bone consolidation and restoration of its function. Bone substitutes are thus primordial in bone reparation surgery. We can distinguish two types of substitutes:
- osteoconductive biomaterials, passive ones, they can receive bone regrowth by vascular and cellular invasion from a receptor tissue
- osteoinductive biomaterials, active ones, inducing a cell differentiation to synthesize mineralized bone matrix
As any biomaterials, bone substitutes have to be adapted to the origin tissue constraints and, in case of the bone tissue, bone implants should stimulate the bone response and have specific mechanical properties.
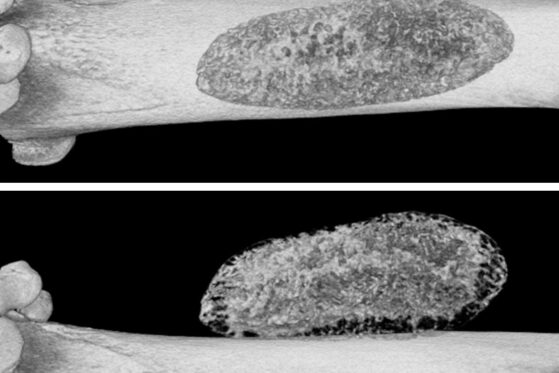
Mineralised biomaterial located beside femur, micro-CT images realised 28 days after implantation – 9µm3 resolution, 45° rotation
In addition to biomaterials for orthopedics applications, Atlantic Bone Screen can also help you to evaluate your biomaterials developed for other applications.
Different kinds of implantations can be performed depending on the goal of the study. Sub-cutaneous (ectopic) implantation of biomaterial can allow to evaluate biocompatibility and host response to the implanted material, assess of the degradation of the material, the tissue integration, or drug release from the loaded biomaterial.
To evaluate biomaterial’s ability to induce bone formation, intermuscular implantation is also commonly used. This allows to evaluate osteoinductivity without mechanical influence, and provides a simpler and more accessible site for implantation.
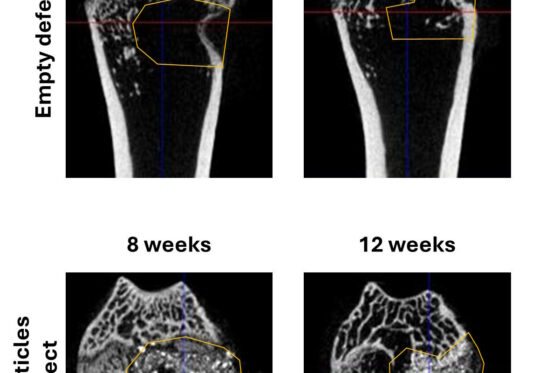
Orthotopic implantation, when biomaterial is implanted in its definitive location, offers more realistic conditions to evaluate biomaterials. This allows to evaluate biomaterials response to its physiological environment, its integration, its mechanical capabilities, and the healing process following the implantation. Implantations can be realised in defects created on calvaria bone, or in femoral condyles.
Once animal studies implemented, our Team can of course manage ex vivo analyses with microCT analysis of implant sites, histology with special staining to highlight inflammation, biocompatibility, tissue regeneration…
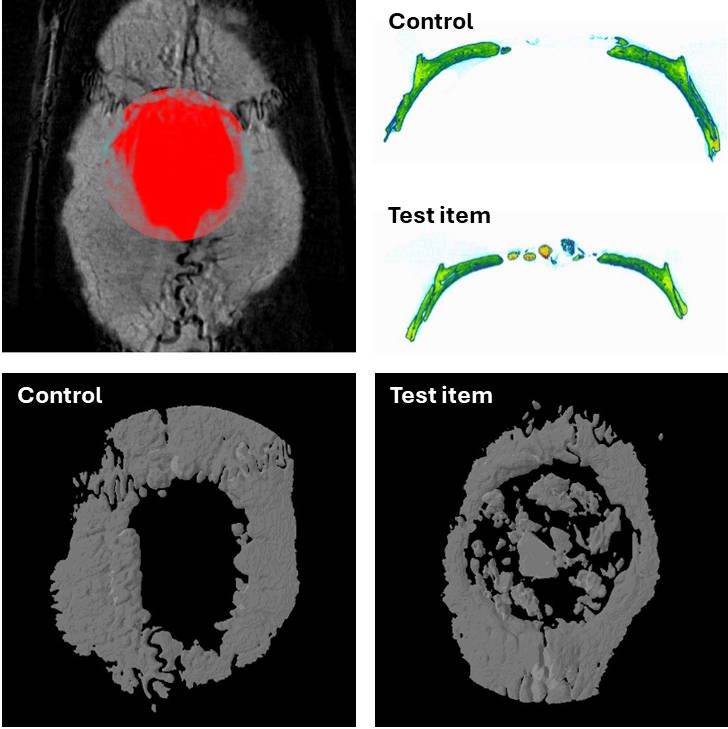
Micro-CT images of a calvarial defect a without (control) and with biomaterial implantation (Test Item)